CYP2C9/VKORC1 Genotypes and Warfarin Sensitivity
Warfarin (Coumadin®) is the most widely prescribed oral anticoagulant in North America and Europe with estimated 2-3 million patients receiving it in the U.S.A. every year, over 20-30 million Rx per year. Warfarin therapy-associated hemorrhage is one of the leading causes of drug-related adverse events, and is the second most common drug after insulin implicated in emergency room visits in the U.S. (43,000 in 2007) according to FDA data. Risk of bleeding, clotting, and stroke in patients taking warfarin is 15%. It is one of the most litigated drugs involved in medical malpractice.
Inter-individual variability in the response to warfarin is partly influenced by genetic variations in two genes; cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) gene. The wide variation in response to warfarin means that dosing is often done on a trial-and-error basis, with the risk of over or under anticoagulating patients until the appropriate dose is found. Hence, the risk of severe bleeding is the highest when the drug is introduced to a patient for the first time. Studies show that such risk is the highest in the first four days after the initial dose as shown in the graph below (Peyvandi et al, 2004. Clinical Pharmacology & Therapeutics. 75:198-203).
.
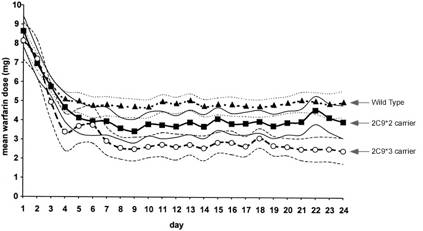
Warfarin dose dependence on CYP2C9 genotypes. Warfarin has a notoriously narrow therapeutic index and a non-linear dose response creating a wide dosing range from 0.5 to 25 mg per day (50-fold) to achieve expected therapeutic outcome (target INR 2-3). A number of factors such as a patient’s age, diet and weight account for a 20% of a dosage variability, whereas a person’s CYP2C9 and VKORC1 together account for 40% of dosage variability. The remaining 40% of dosage variability is still unknown.
FDA Warning. On August 17, 2007 FDA issued a warning to be included on Cumadin® label to determine an individuals CYP2C9 genotype when prescribing warfarin sodium. 
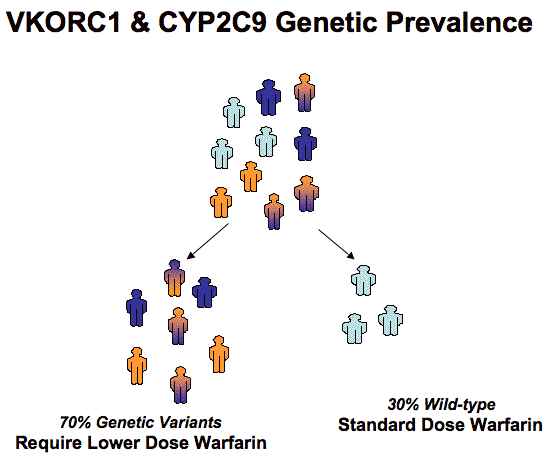
Functional Interaction between warfarin, CYP2C9 and VKORC1. The active component of warfarin is metabolized by cytochrome P450 2C9 (CYP2C9). Up to 35% of the population inherits a form of the CYP2C9 gene variant that results in a CYP2C9 enzyme deficiency. A deficiency in CYP2C9 causes slow metabolism and higher than expected concentrations of the active drug in a patient.1 Increased warfarin concentration in the body increases the risk of bleeding. Warfarin inhibits the formation of active clotting factors by inhibition of vitamin K epoxide reductase complex subunit 1 (VKORC1).2 Inherited variants of VKORC1 increase or decrease the amount of warfarin needed to inhibit the formation of the clotting factors.3
Allele frequency in Caucasians, Africans and Asians:
| Caucasian |
12.5% |
7.0% |
37% |
| African |
3.4% |
1.5% |
14% |
| Asian |
0% |
1.8% |
89% |
| Gage BF, Lesko LJ. J Thromb Thrombolysis. 2008 Feb;25(1):45-51 |
Reporting. Warfarin Genetic Sensitivity test detects and reports on a total of three genetic variants: CYP2C9*2, CYP2C9*3 of cytochrome p450 and (-1639 G>A) in the promoter region of VKORC1 gene. CYP2C9*2 variant reduces warfarin metabolism by 30% to 50% and CYP2C9*3 variant by 90%.4 Variant status (homozygous or heterozygous) as well as a functional phenotype result (normal or slow metabolism and normal or high sensitivity) are reported on.
Dosage computation algorithm. To determine the actual effective therapeutic warfarin dose use the reported genotypes of CYP2C9 and VKORC1 along with the patient’s age, weight, gender, INR range and other characteristics in a dosing algorithm available at no cost to doctors at www.warfarindosing.org.
Reimbursement. Warfarin Sensitivity Tests are reimbursed by major insurance carriers.
Precautions. Periodic INR monitoring is still essential. Numerous factors alone or in combination may influence the response of the patient.
References
- Redman AR, Pharmacotherapy, 2001; 21:235–242.
- Li et al., Nature, 2004; 427:541–544
- Rieder et al., N Engl J Med, 2005; 352:2285–2293
- Gage et al., J Thromb Thrombolysis, 2008; 25(1):45-51
|

|
Information for Laboratories
» Detects the 2 variants in CYP2C9 and 1 variant in VKORC1.
» Can be performed under 2 hours
» Simple setup and interpretation
Click here to see related products.
|